16. Paradoxical regulation and linear amplification¶
(c) 2019 Justin Bois and Michael Elowitz. With the exception of pasted graphics, where the source is noted, this work is licensed under a Creative Commons Attribution License CC-BY 4.0. All code contained herein is licensed under an MIT license.
This document was prepared at Caltech with financial support from the Donna and Benjamin M. Rosen Bioengineering Center.

This lesson was generated from a Jupyter notebook. Click the links below for other versions of this lesson.
import pandas as pd
# Plotting modules
import bokeh.io
import bokeh.plotting
bokeh.io.output_notebook()
Paradoxical regulation: The I1-FFL revisited¶
Let us look back for a moment at an early lesson in which we considered an incoherent feed-forward loop, the I1-FFL, shown below, left. We can abstract the I1-FFL further, wherein X can activate Z and also repress Z with a delay, as shown below, right. The delay is due to the indirect nature of the repression of Z, having to do though the Y intermediary.
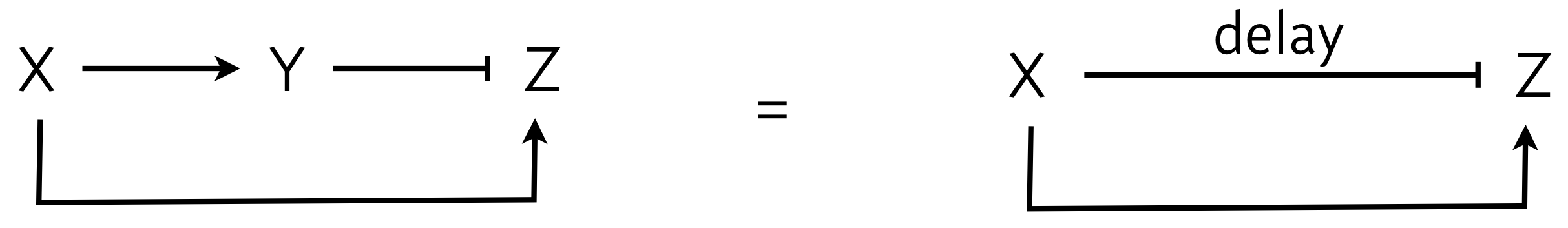
In looking at the figure to the right, we see that X both activates and represses Z. We say that X's action on Y is paradoxical, in that the same gene has opposite regulatory effects on the same target. This stands in contrast to negative feedback loops or two different genes regulating the same target in opposite ways.
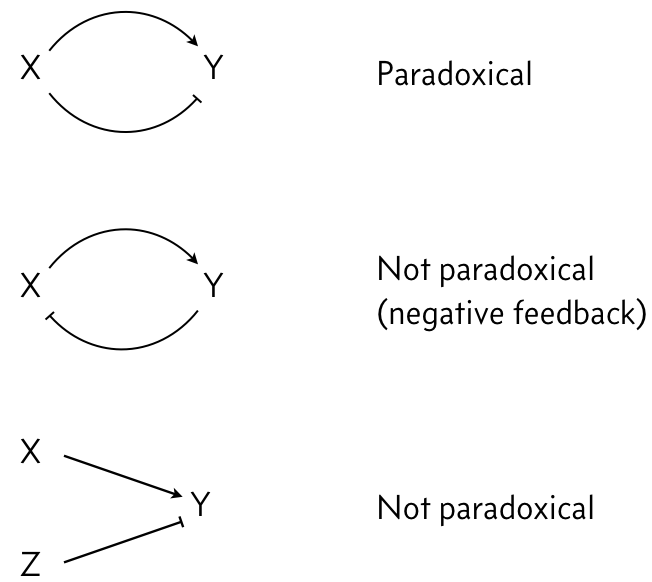
We have already seen properties of the I1-FFL with AND logic.
- It is a pulse generator.
- It has an accelerated response compared to an unregulated circuit.
In this lesson, we will further explore properties of circuits that employ paradoxical regulation, starting with simple two-component signaling circuits like the one shown above.
Two-component paradoxical circuits¶
Two-component circuits are ubiquitous in signaling systems. It makes sense that this is the case; we need minimally two components, a receptor and a signaling molecule (usually a conjunction with a kinase or phosphatase).
First: A two-component non-paradoxical signaling circuit¶
Before considering a circuit with paradoxical regulation, let us consider a simpler circuit wherein a signaling molecule allows the receptor kinase X to phosphorylate of a signaling molecule Y, as shown below.

If we assume the enzymes operate at saturation, we have
\begin{align} \frac{\mathrm{d}y_p}{\mathrm{d}t} = v_1(s)\,x \, y - v_2\,z \, y_p. \end{align}We also have conservation of mass; defining $y_0$ to be the total concentration of Y, we have $y_0 = y + y_p$. Using this expression, we have
\begin{align} \frac{\mathrm{d}y_p}{\mathrm{d}t} = v_1(s)\,x \, (y_0-y_p) - v_2\,z \, y_p. \end{align}We can solve for the steady state concentration of phosphorylated Y, which will affect the expression of the target gene, by setting $\mathrm{d}y_p/\mathrm{d}t = 0$ and solving. The result is
\begin{align} y_p(s) = \frac{v_1(s)\,x}{v_1(s)\,x + v_2\,z}\,y_0. \end{align}This expression reveals a limitation of this circuit. The amount of $y_p$ is obviously dependent on the amount of X, total amount of Y, and amount of Z. We might like the amount of $y_p$ to be dependent only on the concentration of signaling molecule, $s$, and be robust to how much X, Y, and Z are around.
Now: A two-component non-paradoxical signaling circuit¶
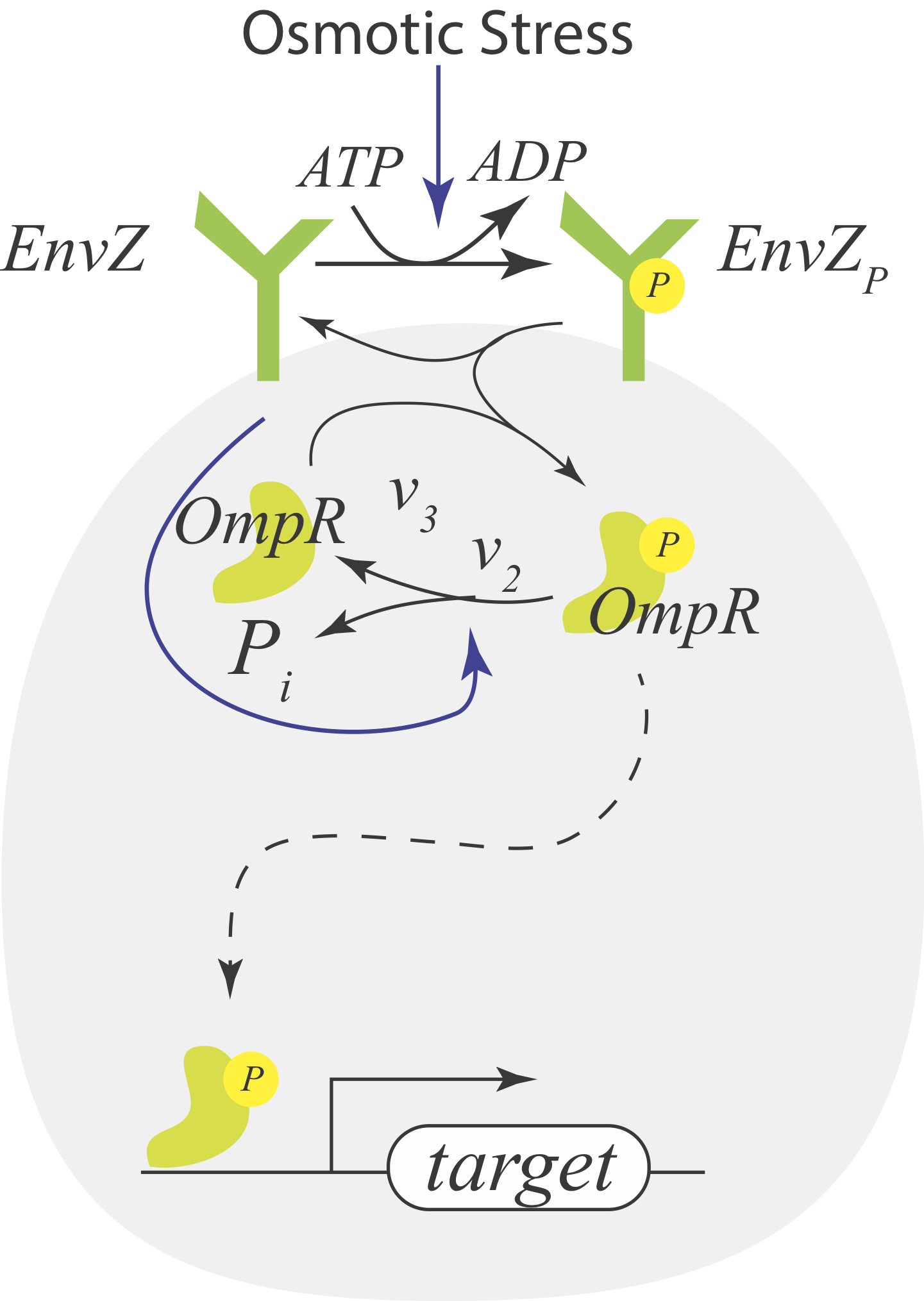
Consider instead a situation where the receptor X is a bifunctional kinase. In its phosphorylated form, it phosphorylates Y via phosphotransfer with rate $v_3$. This is its activating regulation. In its dephosphorylated state, it serves to dephosphorylate Y with rate $v_2$, giving it its paradoxical repressive regulation. So, X paradoxically acts both as a kinase and phosphatase on the same target, Y. It also gets phosphorylated in contact with the signaling molecules S through consumption of an ATP.

We can write out the full dynamics of this system and solve for the steady state, but we can take a shortcut. We can think of the system a black box that takes in ATP and outputs ADP and inorganic phosphate. A phosphate comes in as part of ATP and goes out as inorganic phosphate.
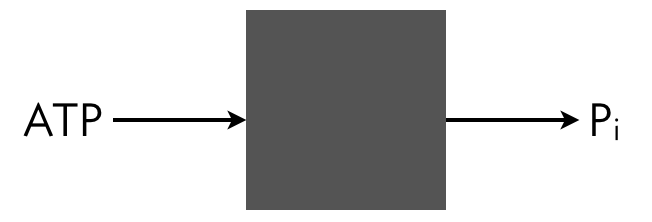
Conservation of mass dictates that the total flux of this phosphate into the system must match the total flux of Pᵢ out of the system. The influx is $v_1(s)\,x$ and the outflux is $v_2\,x\,y_p$. Setting these fluxes to be equal gives
\begin{align} v_1(s)\,x = v_2\,x\,y_p, \end{align}so that
\begin{align} y_p = \frac{v_1(s)}{v_2}. \end{align}This actually is not quite complete, since we still need to respect conservation of mass of Y, so
\begin{align} y_p = \begin{cases} \frac{v_1(s)}{v_2} & y_0 \ge \frac{v_1(s)}{v_2},\\[1em] y_0 & y_0 < \frac{v_1(s)}{v_2}. \end{cases} \end{align}This is dependent only on $s$, and is completely independent of the total amounts of the proteins X and Y. Importantly, the activity, as quantified by the concentration of phosphorylated Y, is linear in the input $v_1(s)$, with a slope of $v_2^{-1}$. Thus, this system, at least before saturating, is a perfect linear amplifier of the input signal.
Key to the linear amplification of signal is the following.
- The cell must produce enough Y to make sure it does not saturate. That is, $y_0$ must be at least $v_1(s_\mathrm{max})/v_2$, where $s_\mathrm{max}$ is the maximum expected concentration of the signaling molecule.
- The gain of the amplifier (the slope of the linear response) is inversely proportional to the dephosphorylation rate $v_2$. Slow dephosphorylation gives a larger gain.
- It is critical that only the dephosphorylated state of the bifunctional kinase X have phosphatase activity. (If the phosphorylated state could act as a phosphatase, the inorganic phosphate balance changes, and we lose the linear amplification feature.)
- All enzymes are operating at capacity. We would lose perfect linear amplification due to nonlinearities if they were not.
- We are neglecting slow reactions, such as spontaneous dephosphorylation, which would also destroy robustness to total protein levels.
An example paradoxical signaling system¶
The EnvZ-OmpR system in E. coli is a classic system that features a bifunctional kinase with paradoxical signaling. It has the same architecture as our example above. EnvZ is a histidine kinase that senses osmotic stress, and OmpR regulates expressin of porin genes in response to osmolarity.
Batchelor and Goulian (PNAS, 2003) introduced a plasmid to E. coli cells where OmpR is under control of the lac promoter. They could then adjust the OmpR levels by titrating IPTG. They could then monitor the activity of the target promoter, which which they engineered to produce fluorescent proteins. The measured the target promoter activity with low osmolarity (minimal media) and with high osmolarity (minimal media with 15% sucrose). The result of their experiment is shown below (using data digitized from the paper).
Over at least an order of magnitude the target promoter activity is robust to OmpR concentration, dependent only on the osmolarity of the surroundings.
Design considerations for two-component paradoxical signaling¶
At the heart of linear amplification by two-component signaling is the bifunctional histidine kinase (labeled X in our generic two-component signaling system). The total concentration X affect the rates of all reaction. Too little X results in a slow response time, but too much X costs cellular resources. So, there is some optimization with respect to the amount of X, depending on the cell's needs. Importantly, if all enzymes are operating at saturation, and the response is fast enough that we can continue to neglect slow reactions like spontaneous phosphorylation, the perfect linear amplification at steady state is robust to the total level of X (and also Y).